PRC: Newcastle launches study investigating new treatment for COPD
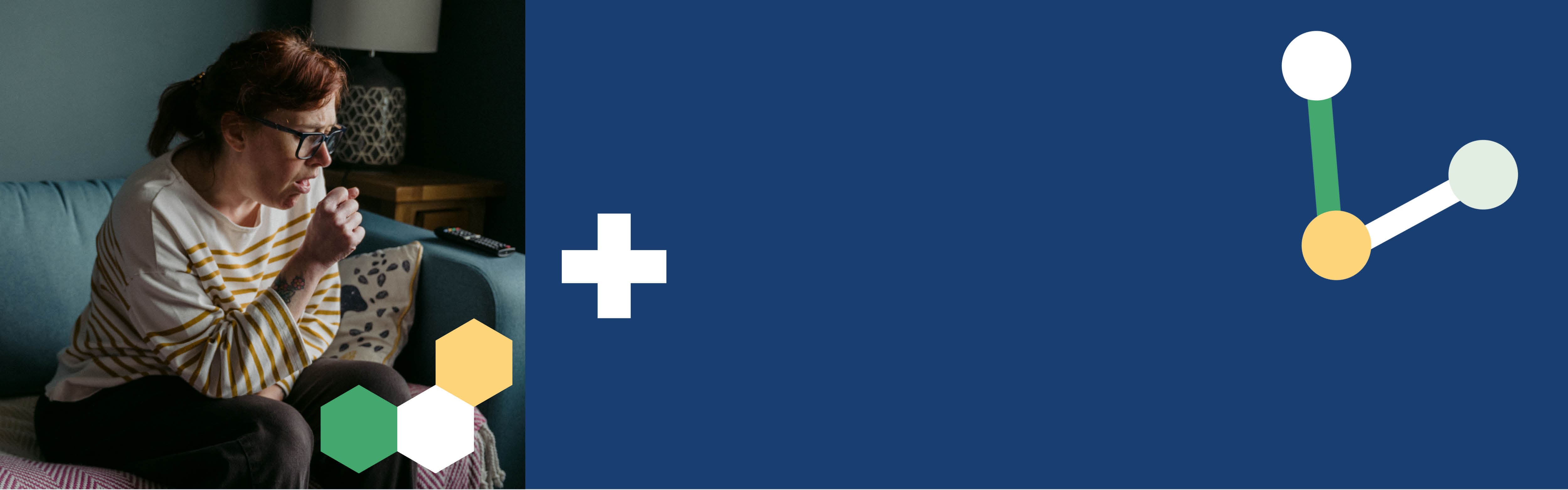
The NIHR Patient Recruitment Centre Newcastle (PRC: Newcastle) is one of five UK sites delivering AERIFY-2, a study looking to investigate a new treatment for people who continue to have COPD symptoms on current medication.
Chronic obstructive pulmonary disease, commonly known as COPD, refers to a large group of lung diseases that are characterised by air flow blockages and breathing problems, such as chronic bronchitis.
An estimated 65 million people have COPD worldwide and, although there is no cure, symptoms can typically be controlled. Currently available therapies for COPD have modest efficacy and/or important safety concerns, which is why research into new treatments is vital.
The purpose of the AERIFY-2 study is to investigate the effect, safety, and tolerability of itepekimab, a drug which is administered by injection to people with COPD who continue to have symptoms on current treatment and who need additional treatment to manage their condition. The study is specifically looking to recruit smokers and former smokers between the ages of 40 and 85.
PRC: Newcastle has a small clinical team consisting of nurses, GPs, consultants and administrative staff who focus on giving participants the best experience possible. Participants get to know the team well over the course of their study visits, which results in a more personal experience.
The facilities at PRC: Newcastle are also designed to deliver the study in a way that is as convenient as possible for study participants. The site is accessible with easy parking, although participants can also choose to travel to their study visits by pre-paid taxi. PRC: Newcastle’s keen focus on the participant experience reduces the likelihood that participants may drop out of the study at a later date - a major benefit for studies with specific recruitment criteria such as AERIFY-2.
Professor Tony de Soyza, Honorary Consultant Respiratory Physician at The Newcastle upon Tyne Hospitals NHS Foundation Trust and Chief Investigator for AERIFY-2, said: “I am delighted that we are offering the AERIFY-2 study to people in Newcastle. COPD is a common condition and symptoms can get worse over time, significantly impacting patients’ quality of life. A benefit of the AERIFY-2 study is that it gives patients the options to complete the treatments at home.
“The aim of the study is to determine whether itepekimab can control symptoms, improve the patient’s breathing, reduce the need for rescue or reliever medications and help them feel better.
“We don’t expect to have any major challenges in delivering AERIFY-2 at PRC: Newcastle. The site has excellent facilities and strong links with patients, local GPs and clinicians. All-in-all we are expecting it to be a smooth process.”
Participants will either be given itepekimab or a placebo, depending on which group they are randomly allocated to. The study will be conducted over the course of 75-77 weeks with around 30 study visits, although some visits may be conducted via telephone.



