Patient recommends participation in clinical trials after major improvement in debilitating lung condition
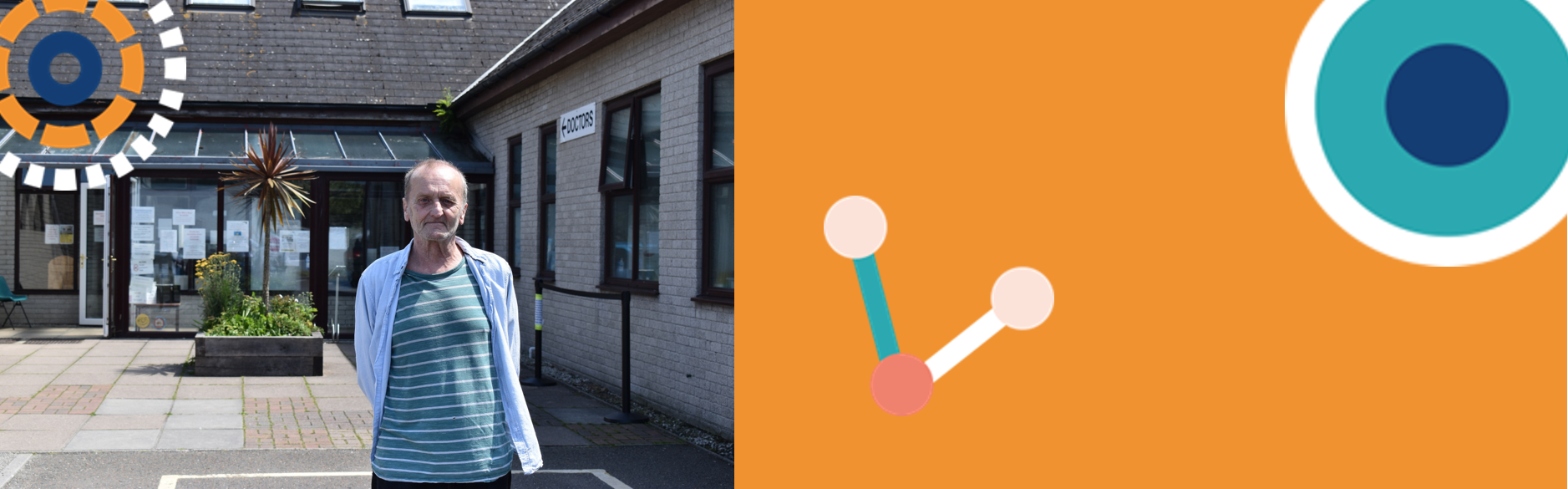
A patient from Cornwall with chronic obstructive pulmonary disease (COPD) is celebrating the ‘incredible’ improvement in his condition linked to taking part in a clinical trial of a new treatment.
Andrew Price from Newquay volunteered for the Bisoprolol In COPD Study (BICS), which is investigating whether the drug bisoprolol, a beta-blocker commonly prescribed for heart problems, can reduce flare-ups of COPD. The study is run by the University of Aberdeen, in conjunction with NHS Grampian, and was supported locally by the NIHR Clinical Research Network South West Peninsula.
Mr Price, who was made aware of the study through the team at his GP surgery, Newquay Health Centre, was given the drug and began to see benefits.
He said: “I live in a Victorian house with lots of stairs, and I first noticed the improvement when I realised I could walk all the way up to the top floor and not be out of breath when I got there. Before, I used to have to sit down for a rest.
“The difference is incredible, now I can walk to all kinds of places I struggled to get to before. For example the doctors surgery, Google Maps says it’s a 16 minute walk from my house and I can do it in 23, which I reckon is pretty good.
“I must admit when I first started I thought it might be a bit of a waste of time, but I’m very positive about it now, and I would definitely recommend taking part in a trial to anyone else.”
BICS is a double-blind trial, meaning that neither patients nor the research team know who is receiving bisoprolol and who is receiving a placebo – a harmless substitute. Mr Price initially took the drug for around 12 months, during which he was monitored by staff at the health centre through extra face-to-face and telephone appointments.
When he came to the end of the 12-month period and began to gradually reduce the dose of the drug, the improvements Mr Price had seen also lessened. So his GP, Dr Nick Jacobsen, asked the central study team whether Mr Price had been taking Bisoprolol or placebo. Because of the marked improvement he had experienced, the team made the unusual decision to let Mr Price know he had been given Bisoprolol as part of the trial, leading Dr Jacobsen to prescribe the drug ‘off-licence’ to treat the COPD.
Bisoprolol is currently only licensed by the Medicines and Healthcare products Regulatory Agency (MHRA) for the heart conditions hypertension, heart failure and angina.
Dr Jacobsen is the NIHR Primary Care Research Lead for Cornwall, and alongside Research Nurse Keren Northcott, is responsible for managing the health centre’s participation in clinical trials.
He said: “We love taking part in research, because as well as giving our patients the opportunity to try new drugs, it allows us to provide a really good service and spend more time with them outside our normal NHS work.
“In Mr Price’s case, the trial drug had a really striking effect, and I’m quite confident this will continue. It still needs to be statistically proven that the effect is seen in enough patients, so there is still a chance that it won’t end up being used routinely to treat COPD. However, Bisoprolol is a very safe drug and one that I’m very familiar with, so I was quite confident prescribing it for COPD in this case.”
Keren Northcott added: “It is really exciting that Mr Price has done so well on the study. Being able to get our patients involved in clinical research makes a difference to their quality of life, and gives them the opportunity to participate in something extra outside of their usual NHS care. That is why I am so passionate about research.”
Recruitment of patients to BICS, which was paused at the start of the COVID-19 pandemic, has now restarted. Results should be published in around two years.



