New figures reveal Patient Recruitment Centre successes
New data from the NIHR shows that the national Patient Recruitment Centres (PRCs) are achieving their study set-up and recruitment ambitions.
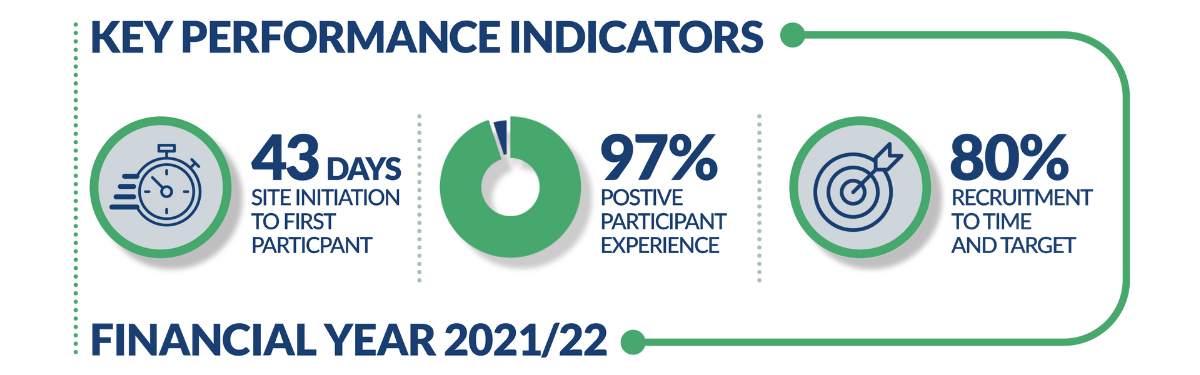
The latest report from financial year 2021/22 revealed that the PRCs initiated commercial studies in a median of 43 days, (against a target of 46 days), and that 80% of the commercial studies they supported achieved their recruitment targets within planned timescales (against a target of 80%).
Sine Littlewood, Director of Business Development and Marketing at the NIHR Clinical Research Network said:
“The PRCs have achieved a number of successes for the life sciences organisations that they have worked with so far. This is especially significant against the backdrop of the pandemic and the challenges experienced by our health and care system. The performance figures show positive signs that the PRCs can and will achieve what they were set up to do - to make it easier and quicker to deliver commercial research in the UK.”
PRCs are 100% dedicated to delivering research funded by the life sciences industry. One of their key aims is to increase the UK’s capacity to deliver commercial clinical research, increasing opportunities for patients to benefit from early access to innovation.
Life science organisations that have benefited from working with the PRCs to date include AstraZeneca, Janssen and Moderna, to name a few.
Feedback from sponsors - where studies closed - said they would consider using the PRCs for commercial research again
All five PRCs opened in 2020, during the first COVID19 pandemic lock down, and while the PRCs were operational during 2020, a full year of performance data was not available until now.
The data, from April 2021 to March 2022 period, has enabled comparison to country-wide performance showing that PRCs are initiating studies faster than the national median for commercial studies, as well as meeting the national ambition of 80% ‘Recruitment to Time and Target’ commercial research.
Recruitment to Time and Target’ is a strict measure. The study must recruit 100% of the target number of participants within the planned recruitment period. One patient below target, or one day over the recruitment period results in a fail.
The site initiation figure is measured as time from the date a site is selected to the date the first participant is recruited.
Key to the success of the PRCs ability to initiate sites quickly is their collaborative operating model, with one costing negotiation for all five sites.
PRC Bradford Clinical Director, Dinesh Saralaya said:
“Our PRCs are really beginning to demonstrate how we can make an impact. During the pandemic our agility was exemplified by our ability to adapt to rapidly recruiting to large-scale COVID-19 vaccine studies. Now, as we move forward, our collaborative working model will make a real difference as our research portfolio expands to a much wider range of therapeutic areas - from Asthma to COPD and IBS to diabetes. We are now refocussing to deliver what we originally set out to do, and we are looking forward to building on our previous successes. “
Feedback from industry and participant experience was wholly positive. From 588 respondents 97% of participants said they would consider taking part in research again. One of the PRCs aims is to to prioritise the patient experience throughout their research journey and responses like this shows the PRCs commitment to achieving this aim.
The PRCs are one of the Sector Deal 2 commitments contributing to the Government’s Life Sciences Industrial Strategy which set out a series of measures to strengthen the UK environment for clinical research and enable growth within the sector. They also feature in the policy paper Saving lives: The Future of UK Clinical Research Delivery and related implementation plans.
For more information visit the PRC webpage.



