Human testing starts on COVID-19 vaccine
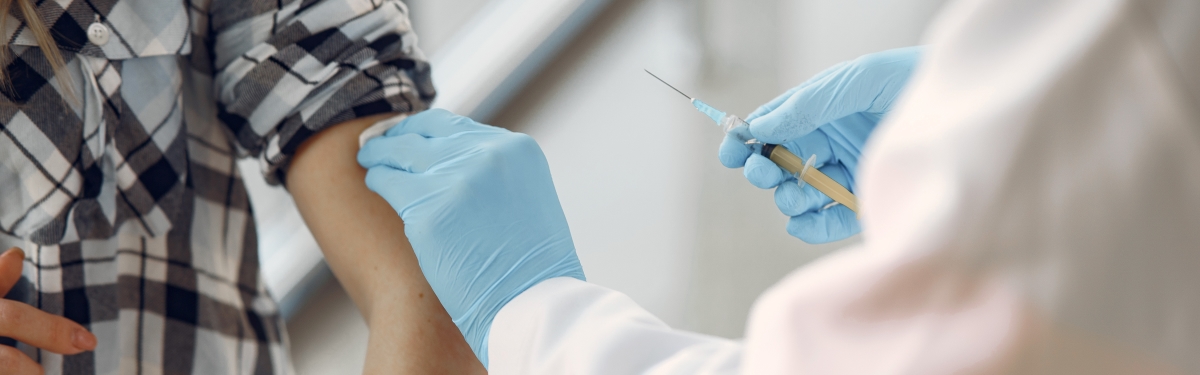
Testing of a COVID-19 vaccine in healthy human volunteers has started in Oxford.
About 1,110 people will take part in the University of Oxford trial with half receiving the vaccine and half receiving a meningitis vaccine.
This is so the results of those receiving the COVID-19 vaccine can be studied in comparison to those who did not.
The NIHR Clinical Research Network Thames Valley and South Midlands is co-ordinating the study across the National Institute for Health Research (NIHR).
The university appealed for volunteers aged 18 to 55 in March to assess whether healthy people can be protected from COVID-19 with the ChAdOx1 nCoV-19 vaccine.
It will also provide information on its safety and ability to generate good immune responses against the virus.
The study will be conducted in Oxford, Southampton, London and Bristol with participants randomly allocated either vaccine. They will not be told which they received until the trial’s end, to prevent bias.
They will keep a record of their health and attend follow-up visits, including providing a blood sample.
The MenACWY meningitis vaccine is being used as a comparison instead of a saline placebo ‘dummy’ vaccine because the ChAdOx1 nCoV-19 may result in minor side effects such as a sore arm, headache and fever, which saline does not.
If saline was used and participants developed side effects, they would know they had the ChAdOx1 nCoV-19 vaccine, which could bias the results. Overall results are expected in two to six months.