Bolton staff and patients play key role in Delta variant COVID-19 research

Patients at Bolton NHS Foundation Trust played a key early role in furthering global understanding of the emerging COVID-19 Delta variant by taking part in groundbreaking research.
The Bolton borough experienced a surge in cases of the Delta variant, also known as the Indian variant, earlier this year.
As positive cases began to rise, the team behind the ISARIC Clinical Characterisation Protocol (CCP) sought to understand more about the new strain and requested further patient samples from the Research and Development (R&D) team at Royal Bolton Hospital.
The CCP is a national study which is playing an integral part in the coronavirus response by coordinating the rapid collection of COVID-19 patient data and biological samples. The study has informed government policy and been responsible for numerous discoveries in the fight against COVID-19.
The Bolton R&D team has been delivering a range of coronavirus research studies, including CCP, on the National Institute for Health Research (NIHR) portfolio since the start of the pandemic.
They acted swiftly to support the CCP’s request for Delta strain samples and for three weeks during May and June liaised with the trust’s COVID wards and Intensive Care Unit to identify suitable patients and recruit them to the study by consent.
In total, 32 lots of samples were collected from 31 patients who consented to be part of the study. Each lot included six blood tests and four types of nose, throat or mouth swabs, as well as some other bodily samples.
Thanks to the rapid response and selflessness of patients, Bolton NHS Foundation Trust provided some of the earliest samples of the Delta variant for research. Some were distributed worldwide to support international intelligence and help inform the global response to the strain.
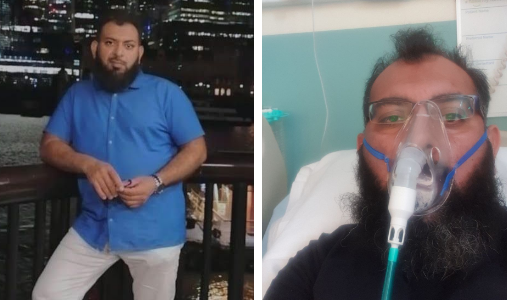
Mohammad Attique (pictured), 40, from Bolton, was among the patients to take part in the study. Ordinarily a fit and healthy man, he fell critically ill with COVID-19 in June this year and spent 22 days in Royal Bolton Hospital, including nine days in ICU - the first six of which he still does not remember. He has since recovered but is still not back to full health and is experiencing shortness of breath.
The father-of-three said: “I was looked after very well and was happy with the treatment I received. We have lost a family member to COVID-19 and I was very happy to be part of this study and contribute to this important research. All of the research staff were very helpful. I was very satisfied with the work and with all staff and colleagues [at Royal Bolton Hospital], including my GP and after care.”
The R&D team at Bolton wished to thank all of the CCP study participants, some of whom such as Mr Attique were critically ill, for demonstrating such altruism by taking part.
Alison Loftus, Research and Development Manager at Bolton NHS Foundation Trust, said: “The whole team at Bolton are very proud to have made significant contributions to this important urgent public health study. National coverage of the local Delta variant outbreak put all eyes on Bolton and the team stepped up and delivered. The commitment and dedication of the Bolton research team along with the amazing contribution from our patients who were willing to have extra tests, despite severe illness, should be celebrated.”
The R&D staff at Bolton NHS Foundation Trust received praise from the CCP study’s Chief Investigator, Professor Callum Semple OBE. He said: “The samples provided should allow us to assess the efficacy of vaccines against the new variant that has emerged. The speed and precision of the R&D team, working hand-in-glove with their clinical colleagues, to provide this critically important material for the national research response is a great example of the benefit of pandemic preparedness. I am immensely grateful to all the staff and patients involved at Bolton.”



