Get involved in COVID19 vaccine research at PRC: Leicester
Why is this study taking place?
This study is taking place to test a vaccine for coronavirus disease-2019 (COVID-19).
The study aims to find out:
1. If the study vaccine helps to reduce how seriously people are affected by COVID-19
2. If the study vaccine is safe for humans
3. More about any side effects the study vaccine may cause
What will I have to do if I take part?
If you choose to take part, these are some of the key things you need to know.
How long the study lasts
You would have to take part for about 2 years and 3 months. This is because you would attend
check-up appointments, which are explained in more detail below.
The injections
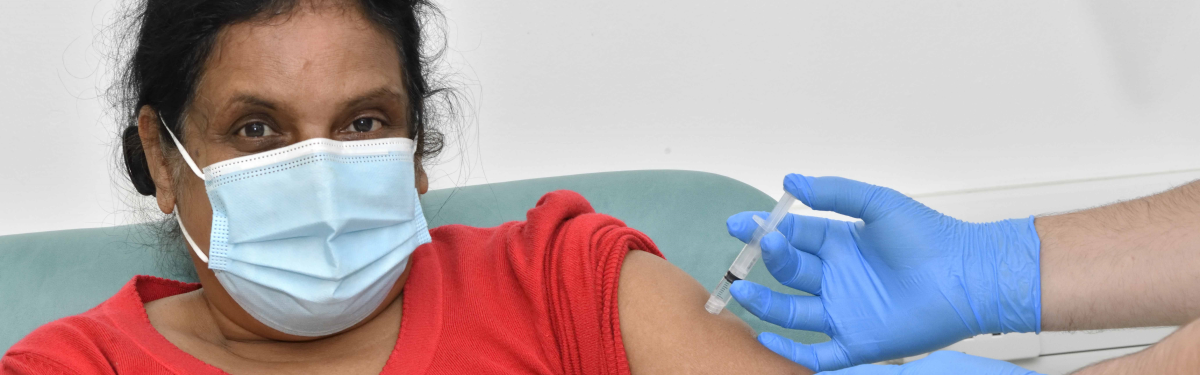
You will be given two injections. One when you first join the study and one about 2 months later.
The injection needle will be put into your upper arm by a doctor or nurse. The injection you receive
will either be the study vaccine or placebo. You cannot catch COVID-19 from the study vaccine or
placebo.
The placebo looks like the study vaccine and is injected in the same way, but it has no vaccine and no
ingredients that have an effect in your body. Neither you nor most of the study team will know
whether you have been given the study vaccine or the placebo.
A computer will randomly decide which injection you receive. You will have a 50% chance of being
given the vaccine or placebo.
You might have some unwanted effects after the injection, because all types of injection can cause:
- Stinging, itching, discomfort, pain, soreness, redness, hardness, bruising or swelling in the area of the arm where you have been given the injection
- Fever and chills
- Rash
- Itching in other areas of your body
- Aches and pains
- Headache
- Dizziness
- Being and/or feeling sick
- Muscle and joint pain
- Feeling very tired
- Fainting
Online questionnaire
To find out more and see if you are eligible please complete an online questionnaire. As part of the study, you will be asked to complete regular online questionnaires which can be done on a mobile device.
- If you do not have a mobile phone or tablet to use, then the team can arrange for you to
borrow a device that will only work for this study. The study team will explain how to
complete the online questionnaire using your mobile device.
Appointments
You will have to go to appointments for different tests and for check-ups with the study team
throughout your time taking part in the study. These appointments might include:
- A blood test to check if you have COVID-19 and how your body is responding to the vaccine
- Answering questions about your health and if you have had any unwanted effects from the injection
- A simple test to check the amount of oxygen in your blood
- A physical exam to check your: height, weight, blood pressure, heart rate and temperature
- A nose swab and giving a saliva (spit) sample
- Filling in a questionnaire about your health on a mobile device
- A pregnancy test, if you are a female who could get pregnant
Some appointments might be a phone call or home visit. The study team will let you know
beforehand.
What happens if I have symptoms that might be due to COVID-19?
- It is important for you to let the study team know if you have any symptoms that might be
due to COVID-19, to help work out if the vaccine works or not. - If you have COVID-19 symptoms, you will be given a pack by the study team containing
swabs to test for COVID-19, a small device to check the amount of oxygen in your blood, and
clear instructions about what to do. - The study team will link the result of your COVID-19 test to the NHS track and trace system.
While you wait for the result, please follow the national Public Health England self-
quarantine guidelines available at that time. The study team will contact you with your result
as soon as it is available, or you will receive a text message from the central NHS system.
FAQs and general information
Can I take part?
Most adults can take part in the study. You can speak to the study team to find out more about
whether you meet the requirements to take part, at the time of booking or during your first
appointment if it is still not clear.
What are the benefits to me?
There are no direct benefits to you, but taking part will help find out if this vaccine can help prevent
COVID-19 and could help others in the future.
Will the visit be safe?
Everyone who takes part in this study will have their temperature taken and will be asked questions
about symptoms when they go to appointments. You will be asked to wear a mask at all times (one
will be provided if you do not have one with you) and all staff will be wearing personal protective
equipment. We have produced a video explaining how we will keep you safe when you visit our
Patient Recruitment Centre: Leicester, which is co-located with the Leicester Diabetes Centre at
Leicester General Hospital.
You may also find it helpful to watch this video produced by the NIHR, which explains what taking part in a COVID-19 vaccine trial is like.
Will taking part stop me from getting COVID-19?
There is no guarantee that the study vaccine will stop you from getting COVID-19. You and most of
the study team will not know if you have been given the study vaccine or the placebo.
Can I bring someone with me to appointments?
Due to COVID-19 restrictions within our hospitals, we can only allow participants to be accompanied if they require a caregiver, who will be asked to wait in one of our waiting rooms to enable social distancing. If you would like to take part in this trial and might require additional support, please let us know.
Can I stop taking part once I have joined the study?
Yes, you can choose to stop taking part at any time for any reason.
Do the injections contain any animal products?
No animal-derived ingredients are in the vaccine or placebo.
If you still have questions or want to find out more, please contact our study team by emailing
ENSEMBLE2_Mailbox@uhl-tr.nhs.uk or calling 0116 258 4499.
