More about our research expertise and experience
At PRC: Leicester we have a wealth of research expertise and experience and a number of significant research successes which set us apart from other sites.
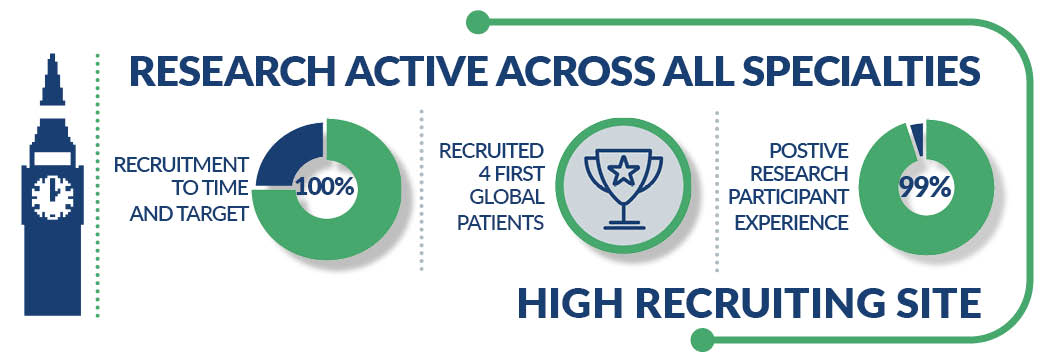
This includes:
- Recruitment to time and target at 100%
- Recruited five first global patients
- 99% of participants say that they would consider taking part in research again
Research expertise
As one of the largest acute trusts in England we have research active staff in all specialties and world-renowned experts in: Cardiovascular, Respiratory, Diabetes, Cancer and Renal Medicine.
Over the last five years approximately 139 investigators have led commercial research in 32 therapeutic areas. When we include non-commercial research that figure increases to 532 investigators covering 57 therapeutic areas, all of whom can be supported by our experienced and highly-skilled team to lead commercial trials.
Track record
In 2021/22, 100% of the commercial trials we delivered recruited to time and target. We are one of the highest recruiting sites in the country, with an average of 52,000 people taking part in research each year over the last five years, 482 of which are commercial studies.
As a result, we have excellent relationships with several pharmaceutical companies including Novartis, Roche and Sanofi, and have partnership agreements with major Contract Research Organisations (CROs) Parexel, PPD and IQVIA.
Recruitment successes
In the last five years, we have recruited four ‘global first’ and one ‘European first’ patients into commercial studies:
- November 2021: First global participant for ILiAD Biotechnologies: A Phase 2b, Multi-Center, Placebo-Controlled, Randomized Study of BPZE1 Intranasal Pertussis Vaccine in Healthy School-Age Children to Assess the Immunological Response and Safety Profile of a Single Dose BPZE1 With and Without Co-Administration of Tetanus, Diphtheria, and Acellular Pertussis (Boostrix™)
- November 2019: First global participant for Genentech Inc study: A Phase 1c Study of Single Dose MTPS9579A in Patients with Asthma.
- January 2019: First European participant for Otonomy Inc study: A prospective, randomised, double-blind, placebo-controlled, multi centre, phase 3 efficacy and safety study of OTO-104 given as a single intratympanic injection in subjects with unilateral Meniere’s disease.
- August 2018: First global participant for GSK study: A double blind (sponsor open) placebo-controlled, stratified, parallel group study to evaluate the efficacy and safety of repeat doses of GSK3772847 in participants with moderate to severe asthma with allergic fungal airway disease (AFAD).
- May 2018: First global participant for Novartis study: An adaptive seamless randomized, double-blind, placebo controlled, dose ranging study to investigate the efficacy and safety of LNP023 in primary IgA nephropathy patients.
Find out more about one of these successes by reading the case study: Using an adaptive model to trial a new treatment for rare kidney disease.
Patient experience
We offer participants on your clinical trial a bright, welcoming and spacious facility with comfortable waiting rooms stocked with refreshments. Our PRC is fully accessible and has both parking and public transport facilities on site. We have a strong culture of public involvement that ensures patients have a say in our decision making. We work closely with the Centre for Black and Minority Ethnic Health to ensure all voices are heard.
In the 2021/22 Participant in Research Experience Survey, 99% of people who took part in research at University Hospitals of Leicester NHS Trust said that they would consider doing so again.
Get in touch
Get in touch to discuss your upcoming research projects and find out how we can help you.
- Phone: +44 (0)7950 893428
- Email: PRCLeicester@uhl-tr.nhs.uk

Read a blog by Clinical Director, Professor Melanie Davies, about myths, misconceptions and major diabetes research happening today.

Recruitment target achieved two months ahead of schedule for the phase trial testing the Janssen COVID19 vaccine candidate.

Case study: medication to reduce the risk of heart disease for people with type 2 diabetes
Find out about a diabetes study for which PRC: Leicester is the joint top recruiting site
