New COVID booster vaccine research study opening at the NIHR Patient Recruitment Centres
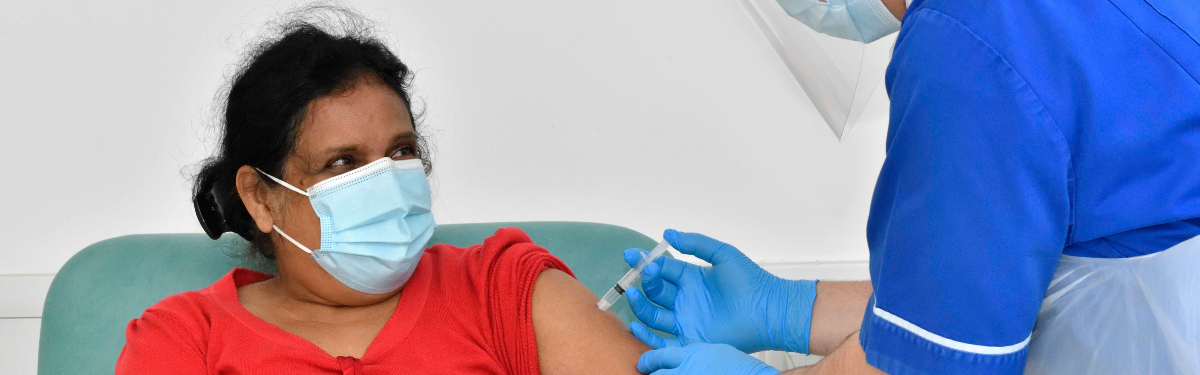
Researchers in Leicester, Bradford, and Exeter are investigating whether a new booster vaccine can provide better protection against the COVID-19 Omicron variant. The study will be run across three of the NIHR patient recruitment centres (PRCs) opening at PRC Leicester, PRC Bradford and PRC Exeter.
The Omicron variant was designated a ‘variant of concern’ by the World Health Organisation (WHO) in November 2021. Commercial study sponsor, Moderna, are hoping an updated vaccine against Omicron is a more effective booster than their authorised vaccine Spikevax, which is based on the original COVID-19 virus detected in late 2019.
Spikevax has shown high levels of efficacy against variants such as Alpha and Delta, and has some protection against Omicron as well. However the mutations in the Omicron variant are distinct enough that researchers suspect an updated version of the vaccine may produce a more robust response from the immune system, which defends the body against infection.
The global study is classed as a phase 3 trial, which means it is comparing a new treatment with the standard treatment currently available. It is expected that up to 4,000 participants will be recruited in the UK.
To take part in the study, participants need to be 16 years of age and older, be in good health, have received either two or three doses of an authorised COVID-19 vaccine, having had their last dose at least three months ago, and have not tested positive for COVID-19 in the last 90 days. Participants will be randomly assigned to receive a single dose of either the new booster vaccine or the currently authorised vaccine, Spikevax.
The study will follow the participants for up to 13 months and involves visits to the NIHR patient recruitment centre at Leicester General Hospital and phone calls from the clinical team.
Professor Adrian Palfreeman, a consultant in infectious diseases at the University Hospitals of Leicester NHS Trust and principal investigator for the study in Leicester, explains: “With new strains of COVID-19 regularly emerging, there is a need to find vaccines to provide greater protection against serious illness.
“At PRC: Leicester, we are keen to ensure that we include as broad a range of people as possible, which is why we are looking for volunteers of all genders from all ethnic backgrounds and of different ages from 16 to the elderly to join the study so we can see if this new booster vaccine provides protection for everyone.
“We have an outstanding track record in delivering COVID-19 vaccine research, which is why we have been selected to run this trial. We are thrilled to bring this opportunity to the people of Leicester and the East Midlands.”
The PRC is one of five in England, dedicated to setting up and delivering late phase commercial clinical trials in the NHS at pace and scale. PRC: Leicester is hosted by the University Hospitals of Leicester NHS Trust.
To find out more about this study and to see if you are eligible to take part, please contact our research teams:
PRC Leicester:
0116 258 8689
PRC Bradford:
01274 383448
PRC Exeter:
01392 406289



