Local MP shares his experience of taking part in a COVID-19 vaccine study in Cornwall
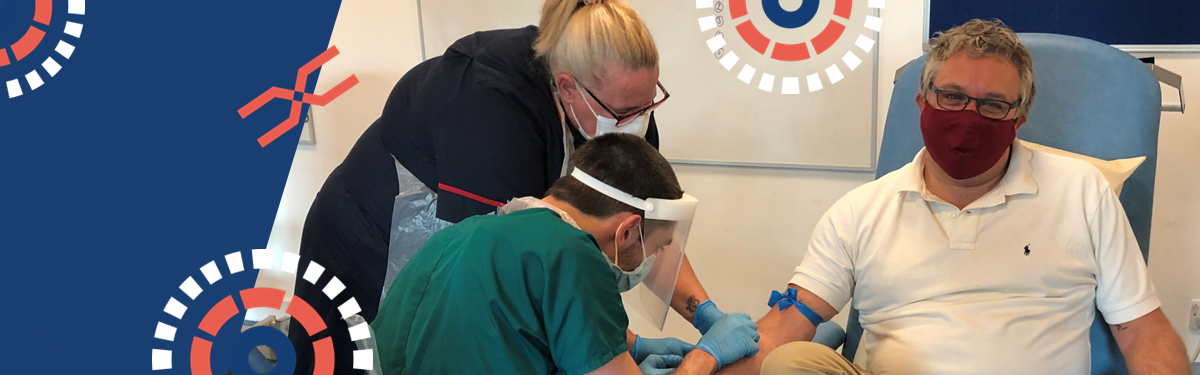
Steve Double is one of the 267 volunteers who took part in a leading phase three COVID-19 vaccine study at Royal Cornwall Hospitals NHS Trust.
The international study, which met its target of 15,000 participants last month, was testing the safety and effectiveness of a promising new vaccine, developed by US biotechnology company Novavax, across a broad spectrum of people, including those from a variety of age groups and backgrounds. It was supported locally by the NIHR Clinical Research Network South West Pensinsula.
Steve, who is an MP for St Austell and Newquay as well as the Parliamentary Private Secretary (PPS) to the Secretary of State for Health and Social Care, agreed to share his experience of what it was like to take part in the trial.
He said: “In my role as PPS to Matt Hancock, I’m aware of just how critical these vaccine studies are for us winning the fight against COVID-19. I wanted to do what I could to play my own small part in this important cause and also help provide some reassurance to people regarding the vaccines.”
“The experience itself was very straight forward. I was provided with all the appropriate information I could need and booked into a slot. On the day the staff were brilliant at explaining everything and answering my questions. After being screened to make sure I was suitable for the study, I received my first shot, after which they monitored me for 30 minutes to ensure I did not have a reaction to it. I returned again last week, after a three week gap, for my second dose.”
The pivotal phase 3 trial opened in the UK on 23 September and is now closed to further recruitment having fully enrolled, ahead of schedule. Recruitment from the South West Peninsula was fantastic with Royal Devon and Exeter NHS Foundation Trust recruiting 545 participants and Royal Cornwall Hospitals NHS Trust recruiting 267 participants – both surpassing their recruitment targets.
Steve added: “To anyone that is thinking of taking part in any of the COVID-19 vaccine studies available locally I would say look at the information available, ask any questions you’ve got, and I would certainly encourage you to participate if you felt it was the right thing for you. I had a very positive experience.”
The government has secured 60 million doses of the Novavax vaccine for the UK, which will be manufactured using FUJIFILM Diosynth Biotechnologies’s facilities in Billingham, Stockton-on-Tees. This will ensure that, once approved by regulators, the vaccine can be supplied as quickly as possible.
Phase 3 studies such as this one involve many thousands of people, giving researchers insights into the effects of a vaccine on a much larger population than phase 1 and 2 studies.



